Abstract
Background: Ataxia telangiectasia mutated and Rad3-related kinase (ATR) mediates cellular response to replication stress and DNA damage. Camonsertib is a potent and selective ATR inhibitor (ATRi) with strong pre-clinical efficacy and promising clinical activity (NCT04497116) in patients with advanced solid tumors with ATRi -sensitizing mutations. However, therapy leads to a rapid dose limiting anemia, implicating potential dysregulation of erythropoiesis. Pre-clinical studies indicate that administering camonsertib for 3 consecutive days followed by a 4 day rest period mitigates anemia without compromising anti-tumor efficacy. High iron requirements are essential for heme synthesis during erythropoiesis, specifically increasing erythroblast vulnerability to iron-dependent reactive oxygen species (ROS) and the potential for iron-mediated oxidative DNA damage. Intracellular iron homeostasis is regulated by a series of proteins involved in iron transport (transferrin receptor 1 (TFR1)) and storage (ferritin) and several iron chaperones both to ferritin (Poly-C binding protein 1 and 2 (PCBP1/2)) and from ferritin (nuclear receptor coactivator 4 (NCOA4)) [Leidgens et al. 2013; Ryu et al. 2017; Mancias et al. 2014]. Iron accumulation in ferritin and NCOA4-mediated ferritinophagy is essential for normal erythropoiesis to occur [Bellelli et al. 2016; Ryu et al. 2017; Santana-Codina et al. 2019]. However, unmitigated release of iron from ferritin increases the sensitivity of cells to ferroptosis, a non-apoptotic iron-dependent form of cell death resulting from iron-mediated lipid peroxidation. We hypothesize that ATRi treatment induces ferroptosis in erythroblasts, thereby contributing to the development of treatment related anemia.
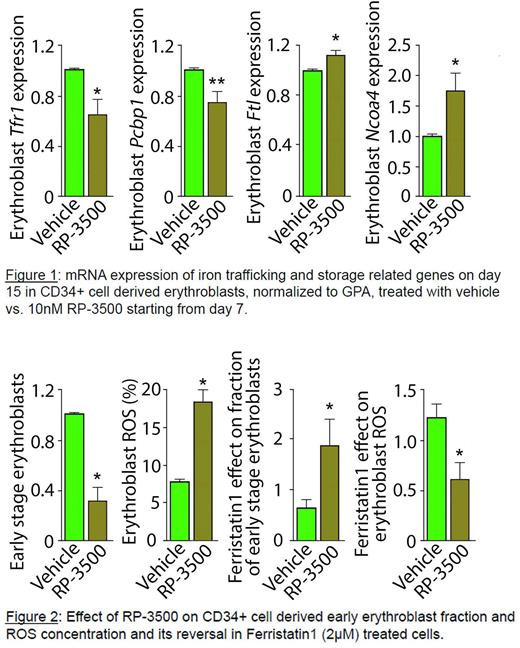
Methods and Results: Using an established protocol to evaluate bone marrow erythroblast differentiation in mice [Liu et al. 2013], we demonstrate that early stage erythroblast depletion occurs 24 hours following camonsertib treatment. This effect is reversible with erythroblast regeneration occurring 2-4 days after treatment is halted. Using an established in vitro protocol for human CD34+ cell differentiation to erythroblasts [Hu et al. 2013], we show that ROS concentration is highest in early-stage erythroblasts (P = 0.002). Next, we demonstrate altered transcripts levels for iron-related genes in erythroblasts with increased ferritin (FTH (P = 0.02) and FTL(P = 0.04)), PCBP1 (P = 0.02), and NCOA4 (P = 0.06) and decreased TFRC (P = 0.06) occur during in vitro erythroblast differentiation (n=5 experiments), confirming reliance on iron trafficking during erythropoiesis. When erythroblasts are treated with camonsertib (1nM, 10nM, and 30nM), proliferation and differentiation are reduced in a dose dependent fashion. Specifically, the proportion of early-stage erythroblasts decreases, while ROS concentration and NCOA4 transcripts increase and FTH transcripts decrease in treated cells; all consistent with enhanced ferritinophagy (Figure 1 and 2). Erythropoietin (EPO) supplementation does not affect erythroblast proliferation, differentiation, apoptosis, or ROS levels in camonsertib treated cells. Finally, the addition of ferristatin-1, a ferroptosis blocking agent, decreases ROS, increases apoptosis, and prevents the dose-dependent reduction in early-stage erythroblast numbers associated with camonsertib therapy, doubling the fraction of early-stage erythroblasts (Figure 2).
Conclusions: These results demonstrate for the first time that 1) camonsertib-induced anemia is reversible in vivo; 2) high levels of ROS, characteristically found in early-stage erythroblasts, increase their susceptibility to ATR inhibition; and 3) the effect of ATR inhibition on erythropoiesis is mediated by increased ferritinophagy, inducing ferroptosis. Although supplemental EPO does not alleviate the erythroid vulnerability to camonsertib, intermittent dosing allows for erythroid cell recovery, minimizing anemia while maintaining antitumor efficacy. Taken together, we provide evidence of the potential for manipulating ferritinophagy and ferroptosis to modulate erythropoiesis and enable an evidence-based strategy to prevent or enhance management of ATRi-induced anemia.
Disclosures
Ferraro:Repare Therapeutics: Current Employment. Li:Repare Therapeutics: Current Employment. Fournier:Repare Therapeutics: Current Employment. Hoffman:Repare: Research Funding; Ionis: Consultancy; Silence Therapeutics: Consultancy; Novartis: Other: Chair DSMB; Protagonist Therapeutics: Consultancy; Turning Point: Research Funding; Scholar Rock: Research Funding; Abbvie: Other: Chair DSMB, Research Funding; Novartis: Research Funding. Fretland:Repare Therapeutics: Current Employment. Roulston:Repare Therapeutics: Current Employment. Ginzburg:Repare: Research Funding; Dexcel: Consultancy; Takeda: Consultancy; Ionis: Consultancy; Protagonist: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal